Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Shibata, Hiroki; Hayashi, Hirokazu; Koyama, Tadafumi*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 83(7), p.532 - 536, 2015/07
Times Cited Count:1 Percentile:1.67(Electrochemistry)The electrochemical properties of curium in a LiCl-KCl eutectic melt were studied in the temperature range of 718-823 K. A small electrochemical cell used in this study was designed for the electrochemical measurement with a small amount (1-20 mg) of the highly radioactive minor actinides contained in molten salts achieved in a hot cell. Our data of apparent standard potentials of a Cm /Cm couple are reasonably in agreement with Osipenko's data (2011) and are lower than Martinot's data (1975). The validity of our data and the reported apparent standard potentials were discussed.
/Cm couple are reasonably in agreement with Osipenko's data (2011) and are lower than Martinot's data (1975). The validity of our data and the reported apparent standard potentials were discussed.
Okamoto, Yoshihiro; Nakada, Masami; Akabori, Mitsuo; Komamine, Satoshi*; Fukui, Toshiki*; Ochi, Eiji*; Nitani, Hiroaki*; Nomura, Masaharu*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 81(7), p.543 - 546, 2013/07
Times Cited Count:7 Percentile:17.93(Electrochemistry)The molten state of simulated high-level waste glass and the behavior of ruthenium element in the melt were investigated by using synchrotron radiation based X-ray imaging technique. Melting, generating and moving of bubbles, condensation and sedimentation of ruthenium element were observed dynamically in continuous 12-bit gray-scale images from the CCD camera. X-ray intensity was obtained easily by digitizing gray-scale values in the image. The existence of ruthenium element is emphasized as a black color in the CCD image at X-ray energy higher than the Ru K-absorption edge. Position sensitive imaging X-ray absorption fine structure (XAFS) measurement was also performed to clarify the chemical state of ruthenium element in the melt.
 Ti
Ti O
O (110) film electrode for lithium batteries
(110) film electrode for lithium batteriesKim, K.-S.*; Tojigamori, Takeshi*; Suzuki, Kota*; Taminato, So*; Tamura, Kazuhisa; Mizuki, Junichiro; Hirayama, Masaaki*; Kanno, Ryoji*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 80(10), p.800 - 803, 2012/10
Times Cited Count:12 Percentile:29.96(Electrochemistry)Electrochemical properties and structure changes of nano-sized Li Ti
Ti O
O during lithium (de)intercalation wereinvestigated using a two-dimensional thin film electrode. Li
during lithium (de)intercalation wereinvestigated using a two-dimensional thin film electrode. Li Ti
Ti O
O thin films were deposited on a Nb:SrTiO
thin films were deposited on a Nb:SrTiO (110)substrate by a pulsed laser deposition technique. In situ X-ray diffraction measurements clarified the drastic structural changes of the Li
(110)substrate by a pulsed laser deposition technique. In situ X-ray diffraction measurements clarified the drastic structural changes of the Li Ti
Ti O
O film upon soaking in the electrolyte and during the first intercalation and deintercalation processes. The surfaceregion of Li
film upon soaking in the electrolyte and during the first intercalation and deintercalation processes. The surfaceregion of Li Ti
Ti O
O had a different structure from the bulk during electrochemical cycling and could cause the nanosizedLi
had a different structure from the bulk during electrochemical cycling and could cause the nanosizedLi Ti
Ti O
O electrodes to have high capacities and poor stabilities.
electrodes to have high capacities and poor stabilities.
Kitawaki, Shinichi; Sakamura, Yoshiharu*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 79(12), p.975 - 976, 2011/12
no abstracts in English
Yasuda, Ryo
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 79(11), p.912 - 913, 2011/09
no abstracts in English
Yasuda, Ryo; Shiozawa, Masahiro*; Katagiri, Masaki*; Takenaka, Nobuyuki*; Sakai, Takuro; Hayashida, Hirotoshi; Matsubayashi, Masahito
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 79(8), p.614 - 619, 2011/08
Times Cited Count:1 Percentile:2.43(Electrochemistry)Nuryanthi, N.*; Yamaki, Tetsuya; Koshikawa, Hiroshi; Asano, Masaharu; Enomoto, Kazuyuki; Sawada, Shinichi; Maekawa, Yasunari; Voss, K.-O.*; Trautmann, C.*; Neumann, R.*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 78(2), p.146 - 149, 2010/02
Times Cited Count:6 Percentile:13.71(Electrochemistry)Poly(vinylidene fluoride) (PVDF) membranes with conical and cylindrical nanopores were prepared in a controlled manner by the ion-track technique, which involved heavy ion beam irradiation and the subsequent alkaline etching. Etching behavior mainly depended on energy deposition of ion beams, and thus its depth distribution, estimated by theoretical simulation, was successfully applied to control the shapes and diameters of the etched pores. Scanning electron microscopy (SEM) and electrolytic conductometry then gave an insight into critical experimental parameters. Interestingly, applying a higher voltage to the conductometry cell promoted track etching up to breakthrough probably because electrophoretic migration of dissolved products occurred out of each pore.
Kofuji, Hirohide; Amamoto, Ippei; Sasaki, Kazuya*; Yasumoto, Masaru*; Takasaki, Yasushi*; Myochin, Munetaka; Terai, Takayuki*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 77(8), p.597 - 600, 2009/08
Times Cited Count:0 Percentile:0.01(Electrochemistry)The process flow of the phosphate conversion technique has been developed for the reduction of high-level radioactive waste (HLW) generated from the metal-electrorefining process. In this study, the results of thermodynamic calculations for the phosphate conversion reactions were examined by the basic experiments. The chlorides of rare earth elements (REE) turned out to be converted into phosphates easily. Furthermore, as the additive for the phosphate conversion reaction, high temperature behavior of lithium phosphate was evaluated to elucidate the thermodynamic property.
Hayashi, Hirokazu; Shibata, Hiroki; Akabori, Mitsuo; Arai, Yasuo; Minato, Kazuo
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 77(8), p.673 - 676, 2009/08
Times Cited Count:7 Percentile:17.19(Electrochemistry)R&D of the nitride fuel cycle technology is underway at Japan Atomic Energy Agency (JAEA). Behavior of americium (Am) in the pyrochemical process, which includes anodic dissolution of AmN and recovery of Am into a liquid cadmium (Cd) cathode by electrolyses in LiCl-KCl eutectic melts, and nitride formation of Am recovered in liquid Cd, is presented.
 /U
/U and U
and U /U couples in molten LiCl-RbCl eutectic
/U couples in molten LiCl-RbCl eutecticNagai, Takayuki; Uehara, Akihiro*; Fujii, Toshiyuki*; Shirai, Osamu*; Sato, Nobuaki*; Yamana, Hajimu*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 77(8), p.614 - 616, 2009/08
Times Cited Count:14 Percentile:31.08(Electrochemistry)Formal redox potentials of the U /U
/U and U
and U /U couples in molten LiCl-RbCl eutectic were determined by cyclic voltammetry. These redox potentials were more negative than those in the LiCl-KCl eutectic but positive comparing to those in the NaCl-CsCl eutectic. This relation would be correlated with the averaged alkali cation radius.
/U couples in molten LiCl-RbCl eutectic were determined by cyclic voltammetry. These redox potentials were more negative than those in the LiCl-KCl eutectic but positive comparing to those in the NaCl-CsCl eutectic. This relation would be correlated with the averaged alkali cation radius.
Toda, Taro*; Maruyama, Takehiro*; Moritani, Kimikazu*; Moriyama, Hirotake*; Hayashi, Hirokazu
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 77(8), p.649 - 651, 2009/08
Times Cited Count:8 Percentile:19.4(Electrochemistry)The distribution coefficients of Am and Ce were measured in the eutectic LiCl-KCl/liquid Ga system at 773K. By using ZrCl as the oxide ion scavenger in order to avoid the formation of such oxychlorides as MO
as the oxide ion scavenger in order to avoid the formation of such oxychlorides as MO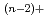 , the effect of oxide ion concentration was well controlled on the distribution coefficients of Am and Ce. The separation factor between Am and Ce was then obtained to be about 100. By comparing the present value with the other experimental and the predicted ones, it was confirmed that the Ga system was more selective than the Bi and Cd system.
, the effect of oxide ion concentration was well controlled on the distribution coefficients of Am and Ce. The separation factor between Am and Ce was then obtained to be about 100. By comparing the present value with the other experimental and the predicted ones, it was confirmed that the Ga system was more selective than the Bi and Cd system.
 Sr
Sr )MnO
)MnO and (Ba
and (Ba Sr
Sr )(Co
)(Co Fe
Fe )O
)O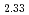 through rietveld refinement and MEM analysis
through rietveld refinement and MEM analysisIto, Takanori*; Shirasaki, Saori*; Fujie, Yoshinori*; Kitamura, Naoto*; Idemoto, Yasushi*; Osaka, Keiichi*; Hirosawa, Ichiro*; Igawa, Naoki
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 77(2), p.161 - 168, 2009/02
Times Cited Count:3 Percentile:8.22(Electrochemistry)We analyzed the mechanism of mixed conduction due to electrons and oxygen ions in (La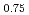 Sr
Sr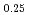 )MnO
)MnO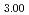 and (Ba
and (Ba Sr
Sr )(Co
)(Co Fe
Fe )O
)O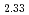 through the Rietveld refinement and maximum entropy method analyses of neutron and synchrotron X-ray diffractions. It was found that Mn-O plane in (La
through the Rietveld refinement and maximum entropy method analyses of neutron and synchrotron X-ray diffractions. It was found that Mn-O plane in (La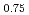 Sr
Sr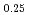 )MnO
)MnO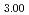 has a strong, isotropic covalent bond that enables conduction of electrons. The (Co, Fe)-O2 plane with a covalent bond and a low concentration of oxygen vacancies in (Ba
has a strong, isotropic covalent bond that enables conduction of electrons. The (Co, Fe)-O2 plane with a covalent bond and a low concentration of oxygen vacancies in (Ba Sr
Sr )(Co
)(Co Fe
Fe )O
)O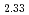 can also conduct electrons. On the other hand, the (Ba, Sr)-O1 plane in (Ba
can also conduct electrons. On the other hand, the (Ba, Sr)-O1 plane in (Ba Sr
Sr )(Co
)(Co Fe
Fe )O
)O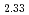 , which has a high concentration of oxygen vacancies, large isotropic displacement parameter, and a strong ionic bond, is responsible for the diffusion of oxygen ions.
, which has a high concentration of oxygen vacancies, large isotropic displacement parameter, and a strong ionic bond, is responsible for the diffusion of oxygen ions.
Hayashi, Hirokazu; Akabori, Mitsuo; Minato, Kazuo; Mizuguchi, Koji*; Kawabe, Akihiro*; Fujita, Reiko*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 75(7), p.528 - 534, 2007/07
Times Cited Count:0 Percentile:0.01(Electrochemistry)The simulation code for the pyrochemical processing of spent nuclear fuels was developed to analyze experimental data, to predict experimental results, and to propose adequate conditions and processes. The Simulation code for Pyrochemical Reprocessing (SPR) is based on calculations of chemical equilibrium and electrochemical reactions. The code also includes the calculations of the current-potential distribution between the electrodes. Some calculations were made to simulate the experimental results on the electro-codeposition process of UO and PuO
and PuO . The phenomena of the redox reactions between Pu
. The phenomena of the redox reactions between Pu and Pu
and Pu ions and those between Fe
ions and those between Fe and Fe
and Fe ions were theoretically analyzed; these redox reactions cause the low current efficiency in the electro-codeposition process. The calculated current-potential distribution around the cathode corresponds to the observed distribution of the oxide deposited on the cathode.
ions were theoretically analyzed; these redox reactions cause the low current efficiency in the electro-codeposition process. The calculated current-potential distribution around the cathode corresponds to the observed distribution of the oxide deposited on the cathode.
Yamaki, Tetsuya; Kozone, Yuichi*; Hiroki, Akihiro; Hosoi, Katsuhiko*; Asano, Masaharu; Kubota, Hitoshi*; Yoshida, Masaru
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 75(2), p.175 - 178, 2007/02
Times Cited Count:10 Percentile:22.72(Electrochemistry)Proton exchange membranes for use in fuel cells were prepared our original ion-track technology, which involves (1) the swift heavy ion irradiation of polyvinylidene fluoride films and subsequent chemical etching to obtain cylindrical pores, and (2) the filling of proton-conducting polymer chains into the etched pores by  -ray-induced graft polymerization. We found that the membranes possessed one-dimensional straight proton conducting pathways parallel to the ion-beam incident axis. Such restricted structures probably led to less water uptake and lower methanol permeability compared to a commercially-available Nafion membrane.
-ray-induced graft polymerization. We found that the membranes possessed one-dimensional straight proton conducting pathways parallel to the ion-beam incident axis. Such restricted structures probably led to less water uptake and lower methanol permeability compared to a commercially-available Nafion membrane.
 with alkali chlorides
with alkali chloridesOkamoto, Yoshihiro; Yaita, Tsuyoshi; Minato, Kazuo
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 73(8), p.745 - 747, 2005/08
The structural change of molten PbCl by mixing with alkali chlorides was investigated by Pb L
by mixing with alkali chlorides was investigated by Pb L -edge XAFS measurements. By the mixing, the nearest neighbor Pb-Cl distance and its coordination number increased, while the Debye-Waller factor and the 3rd cumulant decreased. It is concluded that the coordination structure (PbCl
-edge XAFS measurements. By the mixing, the nearest neighbor Pb-Cl distance and its coordination number increased, while the Debye-Waller factor and the 3rd cumulant decreased. It is concluded that the coordination structure (PbCl )
) is enhanced by the mixing with alkali chlorides.
is enhanced by the mixing with alkali chlorides.
Otori, Norikazu; Ueno, Fumiyoshi; Furukawa, Tomohiro*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 73(8), p.597 - 599, 2005/08
Raman spectra have been obtained for peroxide ion in Na up to 950 K and in molten NaOH up to 900 K. Drastic changes were observed in the spectra around 780 K, which was able to be attributed to the phase transition between beta and alfa forms. The spectra of peroxide ions in molten NaOH was obtained at 610 K just above the eutectic point and the peak can be observed up to 900 K for the melt containing 20 mol% Na
up to 950 K and in molten NaOH up to 900 K. Drastic changes were observed in the spectra around 780 K, which was able to be attributed to the phase transition between beta and alfa forms. The spectra of peroxide ions in molten NaOH was obtained at 610 K just above the eutectic point and the peak can be observed up to 900 K for the melt containing 20 mol% Na 2O
2O . This shows evidently that peroxide ions is able to be present in molten NaOH. An analysis of the peak position suggests the analogous local structure around peroxide ions with that in the crystal.
. This shows evidently that peroxide ions is able to be present in molten NaOH. An analysis of the peak position suggests the analogous local structure around peroxide ions with that in the crystal.
 Raman spectroscopic observation of corrosion reaction of Fe with Na
Raman spectroscopic observation of corrosion reaction of Fe with Na O
O up to 833 K
up to 833 KOtori, Norikazu; Furukawa, Tomohiro*; Ueno, Fumiyoshi
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 73(8), p.675 - 679, 2005/08
Raman spectra have been obtained for alfa- and beta-NaFeO , Na
, Na FeO
FeO , Na
, Na Fe
Fe O
O , Na
, Na FeO
FeO , and Na
, and Na FeO
FeO from room temperature up to 723 K in a state of powder under an inert atmosphere. The comparison of the spectra showed good applicability of Raman spectroscopy to the in situ identification between these sodium iron double oxides. On the basis of this result, we have investigated corrosion reaction on surface of steel with Na
from room temperature up to 723 K in a state of powder under an inert atmosphere. The comparison of the spectra showed good applicability of Raman spectroscopy to the in situ identification between these sodium iron double oxides. On the basis of this result, we have investigated corrosion reaction on surface of steel with Na O
O powder using in situ Raman spectroscopy. The obtained spectra showed corrosion reaction occurs under 723 K and the corrosion product was identified as Na5FeO4. A reaction mechanism for the corrosion based on the above results was presented that the system of Fe + Na2O2 produces corrosive melt under 723 K so that it spreads over the surface and the corrosion products distribute homogeneously on the surface. The corrosion reaction in homogeneous powder mixture of Fe and Na
powder using in situ Raman spectroscopy. The obtained spectra showed corrosion reaction occurs under 723 K and the corrosion product was identified as Na5FeO4. A reaction mechanism for the corrosion based on the above results was presented that the system of Fe + Na2O2 produces corrosive melt under 723 K so that it spreads over the surface and the corrosion products distribute homogeneously on the surface. The corrosion reaction in homogeneous powder mixture of Fe and Na O
O has also been investigated using Raman spectroscopy with the help of XRD and DTA methods. The corrosion products were identified as the double oxides whose compositions coorespond to the stoichiometric ratio of Na to Fe in the starting materials, while the products from the surface reaction of steel with Na
has also been investigated using Raman spectroscopy with the help of XRD and DTA methods. The corrosion products were identified as the double oxides whose compositions coorespond to the stoichiometric ratio of Na to Fe in the starting materials, while the products from the surface reaction of steel with Na O
O tend to have a spcified composition of Na
tend to have a spcified composition of Na FeO
FeO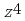 . The differnce can reasonably be explained using the mechanism presented above. It was found from the DTA measurements that Na
. The differnce can reasonably be explained using the mechanism presented above. It was found from the DTA measurements that Na O
O has particularly strong corrosivity for iron, in contrast to Na
has particularly strong corrosivity for iron, in contrast to Na O and NaOH.
O and NaOH.
 and UCl
and UCl in a LiCl-KCl eutectic melt
in a LiCl-KCl eutectic meltKuznetsov, S. A.*; Hayashi, Hirokazu; Minato, Kazuo; Gaune-Escard, M.*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 73(8), p.630 - 632, 2005/08
It was shown that contact of oxide materials with LiCl-KCl eutectic melt containing UCl and UCl
and UCl changes drastically the mechanism of uranium electroreduction. Transformation of voltammograms on addition of oxide ions (Li
changes drastically the mechanism of uranium electroreduction. Transformation of voltammograms on addition of oxide ions (Li O) to the melt LiCl-KCl-UCl
O) to the melt LiCl-KCl-UCl was studied. Similar changes of voltammetric curves were observed during a long contact of oxide materials with LiCl-KCl-UCl
was studied. Similar changes of voltammetric curves were observed during a long contact of oxide materials with LiCl-KCl-UCl molten system due to appearance of oxide ions in the melt resulting from oxide materials corrosion.
molten system due to appearance of oxide ions in the melt resulting from oxide materials corrosion.
Takahashi, Masamitsu
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 72(2), p.128 - 132, 2004/02
no abstracts in English
 O-B
O-B O
O glasses and melts
glasses and meltsUmesaki, Norimasa*; Kita, Y.*; Handa, K.*; Kohara, Shinji*; Suzuya, Kentaro; Fukunaga, T.*; Misawa, M.*; Iida, T.*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 67(6), p.541 - 546, 1999/00
no abstracts in English